Welcome to C-Path Software Engineering Ltd
Professional software consultancy services for start-up and small to medium enterprises. We provide expertise in software development; regulatory compliance, risk management; quality and information security management and computer system validation/software assurance. With a background in Aerospace software engineering and active in software development since the early 1990s, C-Path have experience with a wide range of software and applications for medical devices/IVDs and can provide you with support, advice, training and assistance in medical device product development and the necessary documentation, evidence and data required for successful market approval and release.
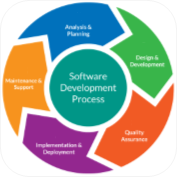
Development
- ISO 62304 SDLC Processes
- Software as a medical device
- Embedded Software
- Clinical Decision Software
- QMS/Non-product Software
- Manufacturing software

Compliance
- UKCA - UK MDR
- EU CE - MDR/IVDR
- FDA 510(k)/PMA/De Novo/CLIA
- Technical Files/Design Dossiers
- ISO 14971 Risk Management
- GAMP 5/Software Validation

Quality
- ISO 13485/MDSAP/ISO 9001
- QMS Implementation
- Risk Management
- Audits
- Support
- Training

Security
- ISO 27001/ISO 27002
- ISMS Implementation
- Security Risk Management
- ISMS Audits
- Medical Device Cybersecurity
- Training
Whether you require support in getting your software to new markets for the first time; assistance with industry regulations, standards and guidance; implementation of management system processes and procedures; or validation of software in your manufacturing processes or quality system; we have the necessary skills and flexibility to meet your project requirements - from a single day's consultancy focused on a particular task or development phase, to management and implementation of a large-scale development program.